Chemical Geometry Chart

Understanding Chemical Geometry: A Comprehensive Guide
Chemical geometry, often referred to as molecular geometry, is the three-dimensional arrangement of atoms in a molecule. This arrangement plays a crucial role in determining a substance’s physical and chemical properties, such as reactivity, polarity, and biological activity. In this article, we will delve into the intricacies of chemical geometry, exploring its fundamentals, classifications, and real-world applications.
Fundamentals of Chemical Geometry
The study of chemical geometry is rooted in the principles of quantum mechanics and molecular orbital theory. Atoms in a molecule are connected by chemical bonds, which can be single, double, or triple bonds. The arrangement of these bonds and the resulting molecular shape are dictated by the electron pair repulsion theory, also known as the Valence Shell Electron Pair Repulsion (VSEPR) theory.
VSEPR Theory: The Cornerstone of Chemical Geometry

VSEPR theory posits that electron pairs surrounding a central atom will repel each other, adopting a geometry that minimizes this repulsion. This theory provides a predictive framework for understanding molecular shapes, allowing chemists to anticipate the geometry of a wide range of compounds.
Key factors influencing molecular geometry include:
- Number of electron pairs (bonding and non-bonding)
- Electronegativity of surrounding atoms
- Molecular symmetry and hybridization
Classifications of Molecular Geometry
Common Molecular Geometries

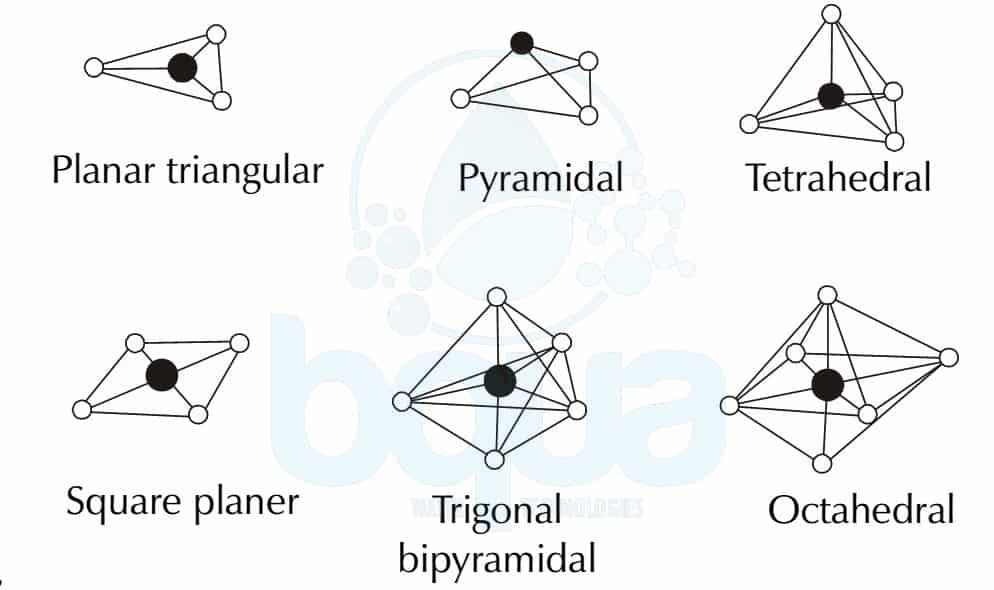
Molecular geometries can be classified into several categories based on the number of electron pairs and their arrangement. Some common geometries include:
| Geometry | Electron Pair Geometry | Examples |
|---|---|---|
| Linear | 2 electron pairs (0 lone pairs) | CO₂, HCN |
| Trigonal Planar | 3 electron pairs (0 lone pairs) | BF₃, SO₃ |
| Tetrahedral | 4 electron pairs (0 lone pairs) | CH₄, NH₄⁺ |
It's essential to note that molecular geometry can be distorted due to factors like lone pair repulsion or differences in electronegativity. For instance, water (H₂O) has a bent geometry due to the presence of two lone pairs on the oxygen atom.
Real-World Applications
Chemical Geometry in Action
Understanding chemical geometry has far-reaching implications in various fields, including:
- Pharmaceuticals: Drug design relies on predicting molecular shapes to optimize binding affinity and selectivity.
- Materials Science: The development of novel materials, such as polymers and composites, requires knowledge of molecular geometry to control properties like strength and flexibility.
- Environmental Chemistry: Predicting the reactivity and toxicity of chemicals involves understanding their molecular geometry and electronic structure.
"The study of chemical geometry is not merely an academic exercise; it's a powerful tool for predicting and controlling the behavior of matter at the molecular level."
Advanced Topics in Chemical Geometry
Isomerism and Stereochemistry
Chemical geometry plays a critical role in understanding isomerism, where compounds with the same molecular formula exhibit different properties due to variations in atomic arrangement. Stereochemistry, the study of spatial arrangements of atoms, is closely tied to molecular geometry and has significant implications in fields like pharmacology and materials science.
Steps to determine molecular geometry:
- Draw the Lewis structure of the molecule.
- Count the number of electron pairs (bonding and non-bonding) around the central atom.
- Apply VSEPR theory to predict the electron pair geometry.
- Consider the effect of lone pairs and electronegativity on the final molecular geometry.
Frequently Asked Questions (FAQs)
What is the difference between electron pair geometry and molecular geometry?
+Electron pair geometry considers all electron pairs (bonding and non-bonding) around the central atom, whereas molecular geometry focuses solely on the arrangement of atoms, excluding lone pairs.
How does hybridization affect molecular geometry?
+Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals, which can influence molecular geometry by altering the arrangement of electron pairs and affecting bond angles.
Can molecular geometry predict a compound's solubility?
+Yes, molecular geometry plays a significant role in determining solubility, as it affects the polarity and intermolecular forces of a compound. For example, linear molecules like CO₂ are nonpolar and insoluble in water, whereas bent molecules like H₂O are polar and highly soluble.
What is the role of chemical geometry in drug design?
+In drug design, understanding molecular geometry is crucial for predicting how a drug molecule will interact with its target, such as a protein or enzyme. This knowledge informs the development of drugs with optimal binding affinity, selectivity, and pharmacokinetic properties.
How does temperature affect molecular geometry?
+Temperature can influence molecular geometry by affecting the vibrational and rotational states of molecules. At higher temperatures, molecules may adopt more disordered arrangements, whereas at lower temperatures, they tend to favor more ordered, symmetric geometries.
Conclusion
Chemical geometry is a fundamental aspect of chemistry, providing insights into the behavior and properties of matter at the molecular level. By understanding the principles of VSEPR theory, molecular classifications, and real-world applications, we can appreciate the intricate relationships between atomic arrangement, molecular shape, and substance characteristics. As our knowledge of chemical geometry continues to evolve, we can expect to see exciting developments in fields like pharmaceuticals, materials science, and environmental chemistry.
Key takeaways:
- Chemical geometry is determined by the arrangement of electron pairs and atoms in a molecule.
- VSEPR theory provides a predictive framework for understanding molecular shapes.
- Molecular geometry has far-reaching implications in fields like pharmaceuticals, materials science, and environmental chemistry.
By mastering the concepts of chemical geometry, we unlock a powerful tool for predicting and controlling the behavior of matter, paving the way for innovative solutions to complex problems.



