5 Tips Half Equivalence Point
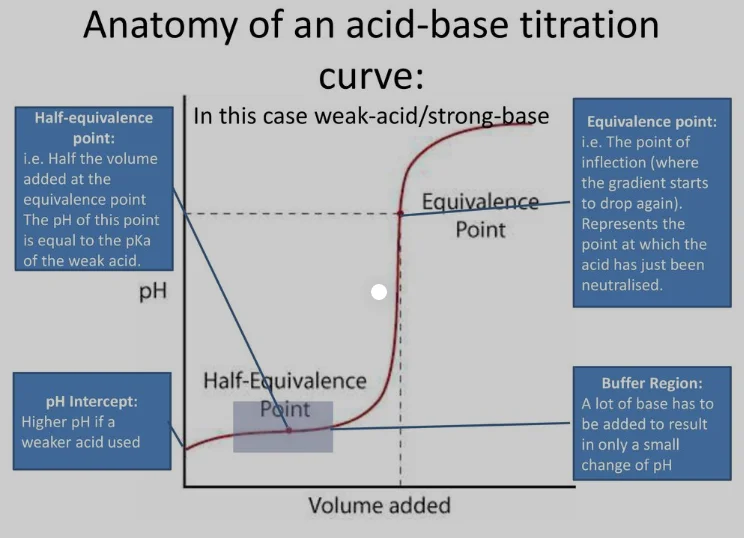
When dealing with acid-base titrations, understanding the concept of the half equivalence point is crucial for accurately determining the concentration of an analyte. The half equivalence point, also known as the half-neutralization point, occurs when the amount of strong acid or base added to a solution is exactly half of the amount needed to reach the equivalence point. At this point, the concentration of the conjugate base (for a weak acid being titrated with a strong base) or the conjugate acid (for a weak base being titrated with a strong acid) is equal to the concentration of the weak acid or base, respectively. Here are five tips to help you work with the half equivalence point effectively:
1. Understanding Buffer Capacity
At the half equivalence point, the solution contains equal amounts of the weak acid and its conjugate base (or weak base and its conjugate acid), forming a buffer solution. This mixture has the highest buffer capacity, meaning it can resist changes in pH upon the addition of small amounts of acid or base. Recognizing the significance of buffer capacity at the half equivalence point is essential for applications in chemistry and biochemistry where maintaining a stable pH is critical.
2. Calculating pH at the Half Equivalence Point
For a weak acid, HA, being titrated with a strong base, the pH at the half equivalence point can be calculated using the Henderson-Hasselbalch equation, pH = pKa + log([A-]/[HA]), where [A-] is the concentration of the conjugate base and [HA] is the concentration of the weak acid. At the half equivalence point, [A-] = [HA], so the ratio [A-]/[HA] = 1, and log(1) = 0. Therefore, the pH at the half equivalence point equals the pKa of the weak acid. This principle is invaluable for predicting and controlling the pH in various chemical and biological processes.
3. Identifying the Half Equivalence Point Experimentally
In a titration curve, the half equivalence point is where the pH starts to rise more sharply as more of the strong base is added (for the titration of a weak acid). It’s before the equivalence point, where the pH rises very steeply. The half equivalence point can be experimentally identified by looking for the point where the addition of a small amount of titrant causes a significant increase in pH, indicating the transition from the buffer region to the steep rise towards the equivalence point.
4. Applying the Concept to Real-World Scenarios
The concept of the half equivalence point has real-world applications, especially in fields like environmental science, pharmaceuticals, and biochemistry. For example, in environmental science, understanding how acid rain interacts with natural buffers in soil and water is crucial. The half equivalence point concept helps predict how much acid can be neutralized before significant pH changes occur, informing strategies for mitigating acid rain effects. Similarly, in pharmaceuticals, the stability and bioavailability of drugs can depend on the pH of their formulation, making the control of pH through buffer systems critical.
5. Interpreting Titration Curves
When analyzing a titration curve, recognizing the half equivalence point is key to interpreting the data correctly. It’s essential to differentiate between the half equivalence point and the equivalence point. The equivalence point, marked by a sharp increase in pH, indicates the complete neutralization of the analyte. The half equivalence point, preceding this sharp increase, provides valuable information about the pKa of the weak acid (or pKb of the weak base) and is crucial for determining the concentration of the analyte and understanding the buffering capacity of the solution.
What is the significance of the half equivalence point in acid-base titrations?
+
How is the pH at the half equivalence point calculated?
+
What are some real-world applications of understanding the half equivalence point?
+Understanding the half equivalence point has applications in environmental science for mitigating acid rain effects, in pharmaceuticals for drug formulation, and in biochemistry for understanding biological buffer systems and maintaining pH homeostasis in living organisms.
In conclusion, the half equivalence point is a crucial concept in acid-base chemistry, offering insights into buffer capacity, pKa/pKb values, and the behavior of weak acids and bases in solution. By understanding and applying this concept, chemists and biochemists can better interpret titration data, predict and control pH in various processes, and develop strategies for real-world applications. Whether in the laboratory, environmental monitoring, or pharmaceutical development, recognizing the significance and implications of the half equivalence point is essential for advancing knowledge and solving complex problems in chemistry and related fields.

