Lewis Dot Structure For At
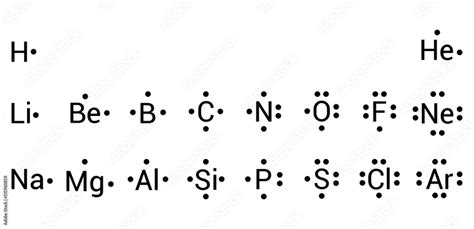
Understanding the Lewis Dot Structure of Argon (Ar)

and Its Implications
In the realm of chemistry, understanding the electron configuration of elements is fundamental. The Lewis dot structure, a visual representation of valence electrons, offers valuable insights into an element’s bonding behavior and reactivity. Today, we delve into the Lewis dot structure of argon (Ar), a noble gas with unique properties.
The Nature of Argon: A Noble Gas
Argon, with atomic number 18, resides in Group 18 of the periodic table, alongside other noble gases like helium, neon, and xenon. These elements are characterized by their full valence electron shells, resulting in remarkable stability and inertness. Electron Configuration and Valence Electrons
Argon’s electron configuration is [Ne] 3s² 3p⁶. This notation reveals that argon has a total of 18 electrons, with the outermost shell (n=3) completely filled with 8 electrons (2 in the 3s orbital and 6 in the 3p orbitals). These 8 valence electrons are crucial for understanding argon’s Lewis dot structure. Constructing the Lewis Dot Structure
The Lewis dot structure for argon is remarkably simple, reflecting its stable electron configuration. Since argon has a full valence shell, it doesn’t require additional electrons to achieve stability. Therefore, its Lewis dot structure consists of the symbol “Ar” surrounded by 8 dots, representing the 8 valence electrons.
Ar:
Implications of Argon’s Lewis Dot Structure
Inertness: The full valence shell makes argon highly unreactive. It rarely forms chemical bonds with other elements, earning its classification as a noble gas.
Non-polarity: With a symmetrical electron distribution, argon molecules are nonpolar. This lack of polarity contributes to its low solubility in polar solvents.
Industrial Applications: Argon’s inertness makes it valuable in various industrial applications, including welding, incandescent lighting, and as a protective atmosphere in food packaging.
Comparative Analysis: Argon vs. Other Noble Gases
While all noble gases share the characteristic of a full valence shell, their Lewis dot structures and properties vary slightly.
| Noble Gas | Lewis Dot Structure | Notable Properties |
|---|---|---|
| Helium (He) | He: | Lightest noble gas, extremely inert, used in cryogenics and balloons. |
| Neon (Ne) | Ne: | Produces orange-red glow in discharge tubes, used in advertising signs. |
| Argon (Ar) | Ar: | Inert shielding gas, used in welding and lighting. |
| Krypton (Kr) | Kr: | Used in energy-efficient light bulbs and laser technology. |
| Xenon (Xe) | Xe: | Heavy noble gas, used in anesthesia and ion engines. |
Historical Context: The Discovery of Argon
The discovery of argon in 1894 by Sir William Ramsay and Lord Rayleigh challenged the prevailing understanding of the periodic table. Its existence as a previously unknown element in air led to the recognition of the noble gas group, filling a crucial gap in the periodic classification.
Future Trends: Exploring Argon’s Potential
While argon’s inertness limits its reactivity, ongoing research explores its potential in specialized applications. Studies investigate its use in:
- Nuclear fusion: As a potential fuel or coolant in future fusion reactors.
- Medical imaging: As a contrast agent in MRI scans.
- Quantum computing: As a medium for trapping and manipulating atoms for quantum information processing.
Key Takeaways
Argon's Lewis dot structure, with its 8 surrounding dots, reflects its full valence shell and inert nature. This simple structure underpins its unique properties, making it a valuable element in various industrial and scientific applications.
FAQ Section
Why doesn't argon form chemical bonds easily?
+Argon's full valence shell of 8 electrons satisfies the octet rule, making it highly stable and uninterested in sharing or gaining electrons to form bonds.
How does argon's Lewis dot structure differ from that of oxygen?
+Oxygen, with 6 valence electrons, requires 2 more electrons to complete its octet. Its Lewis dot structure shows 6 dots around the symbol "O", indicating its need for bonding to achieve stability.
What are some real-world applications of argon?
+Argon is used in welding to shield the weld area from atmospheric contamination, in incandescent light bulbs to prevent filament oxidation, and as a protective atmosphere in food packaging to prevent spoilage.
Can argon be used as a breathing gas?
+While argon is non-toxic, it is not suitable for breathing as it displaces oxygen in the lungs, leading to asphyxiation.
What is the significance of argon's discovery?
+Argon's discovery challenged existing theories about the composition of air and led to the recognition of the noble gas group, a crucial addition to the periodic table.
In conclusion, the Lewis dot structure of argon, with its simplicity and elegance, encapsulates the essence of this noble gas. Its full valence shell, reflected in the 8 surrounding dots, dictates its inertness and unique properties, making it a valuable element with diverse applications across various fields. As research continues, we can anticipate further exploration of argon’s potential, unlocking new possibilities for this seemingly unreactive yet fascinating element.

